Featured
- Get link
- X
- Other Apps
How To Calculate Rate Of Temperature Change
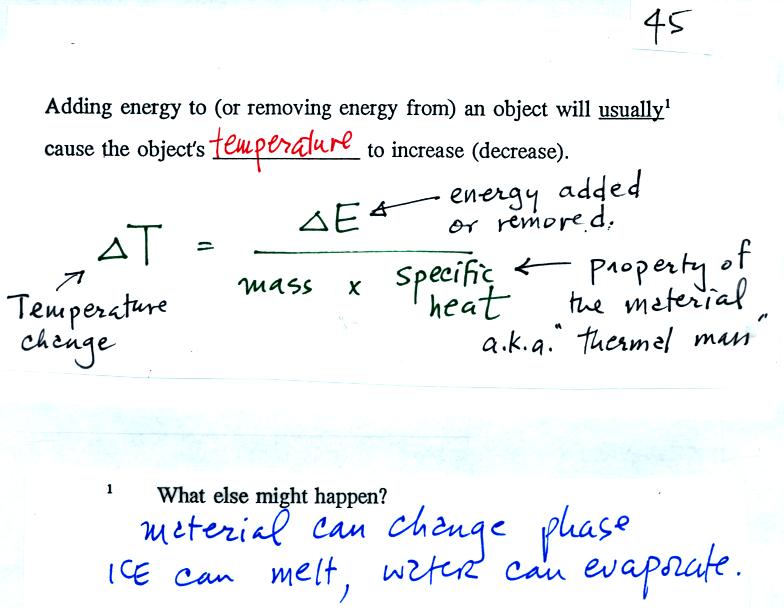
How To Calculate Rate Of Temperature Change. T = mcδt / p. If you have mass flow and a change in temperature then you are a going through a process between two states.
Note that this solution assumes that temperature gradients within the object are negligible. (a) calculate the rate of temperature increase in degrees celsius per second (ºc/s) if the mass of the reactor core is 1.60 × 10 5 kg and it has an average specific heat of 0.3349 kj/kg ⋅ ºc. The thermal expansion coefficients employed are highly dependent on initial temperatures and may undergo significant change.
T = Mcδt / P.
( i) (here the use of minus sign is due to the fact that the temperature is decreasing.) in this ode, t is the time, t ( t) is the temperature at time t and t a is the temperature of the object's environment. Rate of change = (change in quantity 1) / (change in quantity 2) formula 2: The basic formula for the rate of change is:
An Experiment Is Carried Out At A Temperature Of 25 °C.
K = a e − e a r t. Formulas of rate of change in algebra. Empirical data where fresh water is flowing:
< The Calculator Is Appended Here >
Throughout the investigation, remember that: If you have mass flow and a change in temperature then you are a going through a process between two states. (b) how long would it take to obtain a temperature increase of 2000ºc, which could cause some metals holding the radioactive materials to melt?
Fresh Water Flow) Effecting The Natural Rate Of Temperature Change.
T i is calculated numerically by finding a temperature value that satisfies the internal energy balance using equation 19. You can't, without also knowing the mass of the object. Change in temperature = q / cm to calculate the change in.
D T D T = − K ( T − T A).
The thermal expansion coefficients employed are highly dependent on initial temperatures and may undergo significant change. T 1 = 294.43, t 2 = 295. First, it is assumed you have a plot of temperature (t) vs.
Comments
Post a Comment